Genomic Analysis of HCV-Mediated Apoptosis in Cultured Hepatocytes
This study investigates the mechanisms of liver damage associated with HCV infection, focusing on the direct effects of the virus on cell cycle regulation and apoptosis. We performed gene expression profiling on Huh-7.5 cells infected with HCV at multiple time points.

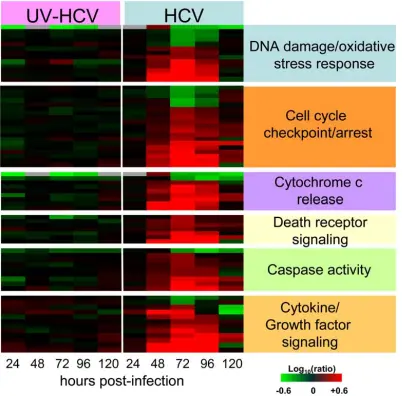
Gene Expression and Apoptosis:
Genes related to cell death were highly induced during peak viral replication, and activated caspase-3 was detected, confirming apoptosis in HCV-infected cells.
Cell Cycle Disruption:
Flow cytometry analysis showed a significant reduction of cells in S-phase in HCV-infected cells compared to control cultures, indicating that HCV infection delays cell cycle progression.
Several genes involved in oxidative stress and TGF-b1 signaling were upregulated, suggesting they contribute to this delay.
In Vivo Correlation:
Similar gene expression changes were observed in liver tissue from HCV-infected, particularly those with rapidly progressive fibrosis, linking the in vitro findings to clinical relevance.
Viral Load and Disease Progression:
The study suggests that higher viral loads correlate with increased liver injury, emphasizing the role of HCV replication in disease severity.
Cell Cycle and Apoptosis Mechanism:
The majority of differentially expressed genes in infected cells were related to cell cycle checkpoints, particularly genes controlling G1 arrest. This supports the idea that HCV infection perturbs cell cycle regulation, contributing to apoptosis.