From stem cell to red cell: regulation of erythropoiesis at multiple levels by
multiple proteins, RNAs, and chromatin modifications
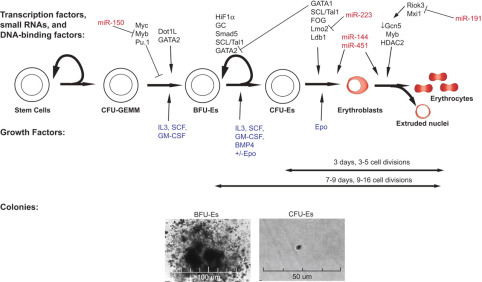
Erythropoiesis is the process by which red blood cells (RBCs) are produced. This occurs in mammals starting with pluripotent stem cells in the fetal liver and continuing in the adult bone marrow. The earliest progenitors in the erythroid lineage are the burst-forming unit-erythroid (BFU-E) cells, which gradually differentiate into colony-forming unit-erythroid (CFU-E) progenitors. These progenitors undergo several stages of differentiation to form mature RBCs.
1. Key Stages of Erythropoiesis
BFU-E to CFU-E Transition: The process begins with the commitment of stem cells to the erythroid lineage, followed by differentiation from BFU-E progenitors into rapidly dividing CFU-E cells.
CFU-E Proliferation: CFU-E progenitors divide several times as they progress through erythropoiesis. This stage involves changes in cell size, chromatin condensation, and the production of hemoglobin.
Enucleation and Maturation: The final stages involve enucleation, where the nucleus is expelled from the cell, resulting in a mature, anucleate RBC.
2. Regulation by Erythropoietin (Epo)
Role of Epo in Erythropoiesis: Erythropoietin (Epo) is a cytokine produced by the kidneys that plays a pivotal role in regulating erythropoiesis. It binds to its receptor on erythroid progenitor cells, promoting their survival, proliferation, and differentiation.
Stages of Dependence on Epo: Initially, CFU-E progenitors are highly dependent on Epo for their proliferation. As differentiation progresses, their reliance on Epo decreases.
3. Role of Other Hormones in Erythropoiesis
SCF, IGF-1, and IL-3/IL-6: In addition to Epo, other hormones like stem cell factor (SCF), insulin-like growth factor 1 (IGF-1), and interleukins (IL-3 and IL-6) contribute to regulating the activity of BFU-E and CFU-E progenitors.
Stress Responses: Under conditions of stress (e.g., hemolysis), the body produces more BFU-E progenitors to increase RBC production, even when Epo levels are high.
4. Transcription Factors in Erythropoiesis
Key Transcription Factors: Several transcription factors are involved in regulating erythropoiesis. Stat5, GATA-1, and Klf1 are crucial for driving the expression of genes necessary for RBC production.
Interaction with Regulatory DNA Regions: These transcription factors bind to enhancer and promoter regions of target genes to activate or repress their expression. This ensures the correct genes are active at the appropriate stage of differentiation.
5. Chromatin Modifications in Gene Regulation
Histone Modifications: Chromatin modifications, such as histone methylation, play a key role in regulating gene expression during erythropoiesis. For example, specific histone modifications mark genes for activation or repression.
Enhancer Regions: Many transcription factors, such as GATA-1, primarily function through binding to distal enhancer regions of genes. This interaction leads to chromatin remodeling, which facilitates gene expression at the correct stage.
6. Role of Extracellular Matrix in Erythropoiesis
Fibronectin and Integrins: During terminal differentiation, the extracellular matrix protein fibronectin helps regulate the final stages of erythropoiesis. Fibronectin interacts with integrins on erythroid progenitors, supporting terminal cell division and enucleation.
Impact of Fibronectin Absence: The absence of fibronectin affects the enucleation process, leading to the formation of larger, less mature RBCs, which may have implications for certain anemias.
Erythropoiesis is a tightly regulated process that involves multiple layers of control, from extracellular signals like Epo to intracellular transcription factors and chromatin modifications. Understanding these regulatory mechanisms is essential for advancing our knowledge of hematopoiesis and could lead to new therapeutic strategies for diseases related to RBC production.
Refer to the PDF for more information.